Accelerating Breakthroughs
Feed the machine. Step on the gas. Deliver the product.

Feed the machine. Step on the gas. Deliver the product.

The journey from idea to breakthrough can be a long one – especially in pediatrics. Many pediatric diseases affect just a small number of children, so gathering sufficient data for research can be challenging. And in their efforts to develop treatments that will benefit the largest number of patients possible, pharmaceutical companies often choose to focus their research and development efforts on drugs and devices for adults.
At Children’s Hospital of Philadelphia, we are finding creative ways – often with the help of our generous philanthropic supporters – to overcome these challenges and get much-needed treatments to patients much faster.
When looking ahead to the next big breakthrough, our team sees possibilities everywhere – and we have programs in place to turn the most promising ideas into solutions that will benefit patients.

Discovery and innovation are at CHOP’s very core. Every day, our brilliant physicians and researchers work relentlessly to advance breakthroughs and drive pediatric scientific knowledge forward.
Each year, the most promising work at CHOP receives the funding needed to reach specific research, diagnostic and therapeutic goals. These trailblazing initiatives are called Frontier Programs.
Frontier Programs allow us to:

CHOP’s 20 Frontier Programs have provided breakthrough, lifesaving care to children from more than 45 states and more than 20 countries. These programs have transformed pediatric care worldwide.
The Cell & Gene Therapy Collaborative (CGTC) aims to cultivate support for more CHOP-led cell and gene therapy research, significantly increase the number of potential new pediatric cell and gene therapies that are the product of CHOP-led research, and accelerate the pace of clinical development in order to provide new therapies to children. The CGTC advances CHOP’s pre-clinical and clinical infrastructure and promotes and fosters continued collaboration and partnership across many areas of the hospital and Research Institute.
At CHOP, we understand the challenges of commercialization, particularly for orphan (rare) diseases. Our internal expertise is critical to our external partnerships. We have the data and in-depth knowledge necessary to fuel future breakthroughs, and we explore all funding avenues, including partnerships with pharma and investments in spinoff companies.
Our deliberate, strategic investments in innovation have paid off big, and we’ve learned a lot along the way. Our work has helped establish Philadelphia as a hub for cell and gene therapy, and we’ve powered some of the most significant advances in this exciting field.
 In August 2017, the FDA approved the use of chimeric antigen receptor T-cell (CAR-T) therapy for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) in children and young adults. Pioneered through CHOP’s collaboration with the University of Pennsylvania and licensed by Novartis, Kymriah is an engineered cell therapy in which a patient’s own reprogrammed T cells locate and kill cancerous cells. The FDA’s landmark approval of this CAR-T cell gene immunotherapy is the result of five years of multisite, global clinical trials supervised by lead investigator Stephan Grupp, MD, PhD, Director of the Cancer Immunotherapy Program at CHOP. The approval of Kymriah has shifted the paradigm for pediatric cancer treatment — and at CHOP, more cell therapies are on the horizon.
In August 2017, the FDA approved the use of chimeric antigen receptor T-cell (CAR-T) therapy for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) in children and young adults. Pioneered through CHOP’s collaboration with the University of Pennsylvania and licensed by Novartis, Kymriah is an engineered cell therapy in which a patient’s own reprogrammed T cells locate and kill cancerous cells. The FDA’s landmark approval of this CAR-T cell gene immunotherapy is the result of five years of multisite, global clinical trials supervised by lead investigator Stephan Grupp, MD, PhD, Director of the Cancer Immunotherapy Program at CHOP. The approval of Kymriah has shifted the paradigm for pediatric cancer treatment — and at CHOP, more cell therapies are on the horizon.
 Almost 20 years ago, Katherine High, MD, then Director of the Center for Cellular and Molecular Therapeutics at CHOP, aspired to using gene therapy in the treatment of inherited retinal dystrophies, a group of eye disorders characterized by retinal degeneration due to two mutations in the RPE65 gene. After a decade of research, High proved the potential of gene therapy in the treatment of retinal dystrophies. In an effort to propel her research and garner the funding and talent necessary to quickly commercialize and deliver this life-changing therapy, CHOP helped found Spark Therapeutics, a Philadelphia biotechnology company. In less than one year, Spark tested its blindness therapy in clinical trials that ultimately illustrated the treatment’s game-changing potential. In 2017, Spark produced Luxturna, the first U.S. Food and Drug Administration-approved gene therapy for an inherited form of blindness. Delivered by subretinal injection, Luxturna provides retinal cells a working copy of the mutated RPE65 gene. This prompts the cells to make the RPE65 protein, which allows the visual cycle to continue and light to be converted to electrical signals that can be interpreted by the brain.
Almost 20 years ago, Katherine High, MD, then Director of the Center for Cellular and Molecular Therapeutics at CHOP, aspired to using gene therapy in the treatment of inherited retinal dystrophies, a group of eye disorders characterized by retinal degeneration due to two mutations in the RPE65 gene. After a decade of research, High proved the potential of gene therapy in the treatment of retinal dystrophies. In an effort to propel her research and garner the funding and talent necessary to quickly commercialize and deliver this life-changing therapy, CHOP helped found Spark Therapeutics, a Philadelphia biotechnology company. In less than one year, Spark tested its blindness therapy in clinical trials that ultimately illustrated the treatment’s game-changing potential. In 2017, Spark produced Luxturna, the first U.S. Food and Drug Administration-approved gene therapy for an inherited form of blindness. Delivered by subretinal injection, Luxturna provides retinal cells a working copy of the mutated RPE65 gene. This prompts the cells to make the RPE65 protein, which allows the visual cycle to continue and light to be converted to electrical signals that can be interpreted by the brain.
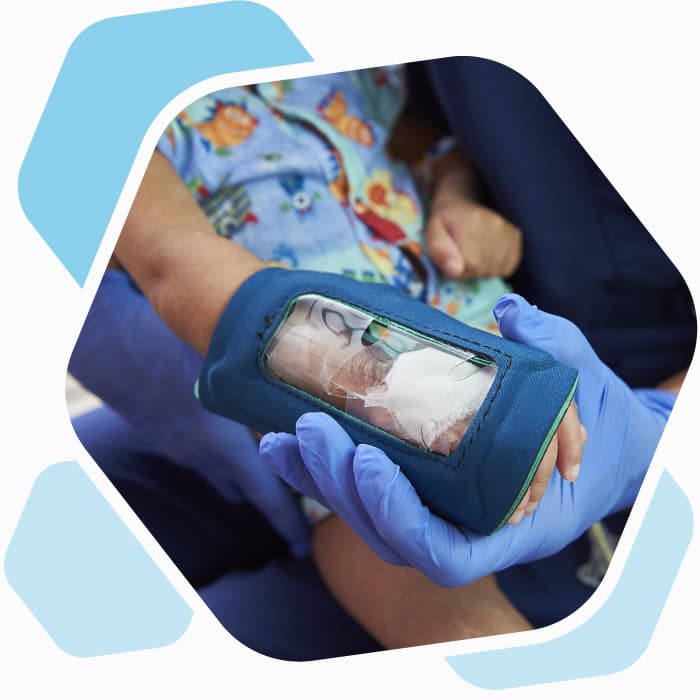 Unlike other protective covers for children with intravenous (IV) lines, the See-IV has a clear plastic window through which a nurse can see the IV area. Designed by Michele Davey, RN, and CHOP collaborators, the See-IV makes frequent IV checks more efficient for staff and less disruptive for patients. With the guidance and support of CHOP’s Office of Entrepreneurship and Innovation (OEI), the team developed and licensed See-IV through Medline, a medical equipment manufacturer.
Unlike other protective covers for children with intravenous (IV) lines, the See-IV has a clear plastic window through which a nurse can see the IV area. Designed by Michele Davey, RN, and CHOP collaborators, the See-IV makes frequent IV checks more efficient for staff and less disruptive for patients. With the guidance and support of CHOP’s Office of Entrepreneurship and Innovation (OEI), the team developed and licensed See-IV through Medline, a medical equipment manufacturer.
Learn More About See-IV and the Office of Entrepreneurship and Innovation
 Diagnostic Driving’s simulated driving software rapidly identifies dangerous driving behaviors, categorizes drivers by crash risk and delivers personalized online coaching. Developed by Flaura Winston, MD, PhD, Founder and Scientific Director of the Center for Injury Research and Prevention (CIRP) at CHOP, and Venk Kandadia, MPH, former CIRP clinical research project manager, Diagnostic Driving’s scientifically-grounded software results from decades of CIRP research on driver safety. With the support of OEI and a seed investment from CHOP, Diagnostic Driving was spun out as a standalone company in 2015.
Diagnostic Driving’s simulated driving software rapidly identifies dangerous driving behaviors, categorizes drivers by crash risk and delivers personalized online coaching. Developed by Flaura Winston, MD, PhD, Founder and Scientific Director of the Center for Injury Research and Prevention (CIRP) at CHOP, and Venk Kandadia, MPH, former CIRP clinical research project manager, Diagnostic Driving’s scientifically-grounded software results from decades of CIRP research on driver safety. With the support of OEI and a seed investment from CHOP, Diagnostic Driving was spun out as a standalone company in 2015.
Our world-changing work is powered by people like you. Help us make our next breakthrough.